We help you
help your client.
Over the past 2 decades, we have worked with great effort to earn our reputation as a market leader in the field of dental implants, known for always pushing the boundaries of innovation.
With a record-breaking number of patents registered, we empower dentists to advance their work, and thereby help more clients achieve beautiful smiles and improved wellbeing.
We have done so with great passion, expertise, and outstanding professionalism.
What do we
Stand for?
Our Credo
Compliant and Certified Company
Cortex Dental Implants Industries Ltd. Quality Management System is certified for ISO13485.








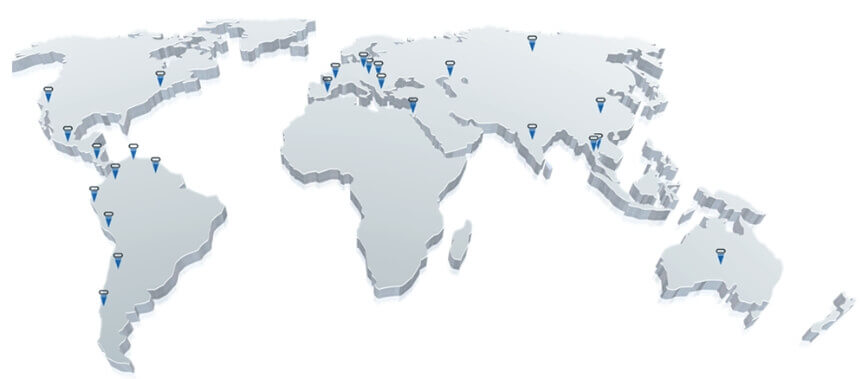
Cortex in a Nutshell
We operate from subsidiaries in China, Latin America, and the U.S.A,
In conjunction with a global network of distributors.
With an entrepreneurship program that includes business mentoring, digital marketing training, clinical and practical education, and creative branding strategy.
MAGIX – A fully-patented, ideal drill-less implant
Guided Surgery Kit – Our novel digital lab provides a fully digital workflow
Digital Dentistry – Cortex CAD/CAM lab using computers, 3D simulation, and printers, optical scanners.
Our People
Questions? We are here for you.